Neurodevelopment, Nutrition and Genetics
Neurodevelopment, Nutrition and Genetics. A contemporary retrospective on neurocognitive health on the occasion of the 100th anniversary of the National Institute of Nutrition, Hyderabad, India
https://www.plefa.com/article/S0952-3278(22)00039-4/fulltext
This article may also be downloaded in PDF format from here: Crawford_PLEFA_2022_preprint.pdf.
Michael A. Crawford1, Yiqun Wang 1, David E. Marsh 1, Mark R. Johnson 1, Enitan Ogundipe 1, Ahamed Ibrahim 3, Hemalatha Rajkumar 3, S. Kowsalya 4, Kumar S.D. Kothapalli 2, J.T. Brenna 2
1 Institute of Brain Chemistry and Human Nutrition, Chelsea and Westminster Hospital Campus of Imperial College, London.
2 Dell Pediatric Research Institute, Dell Medical School, The University of Texas at Austin, 1400 Barbara Jordan Blvd, Austin, Tx 78723
3 National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India.
4 Department of Food Science and Nutrition, Avinashilingam Institute for Home Science and Higher Education for Women (Deemed to be University), Coimbatore, India.
Correspondence: tbrenna@utexas.edu; kkothapalli@utexas.edu
Keywords. brain, nutrition, mental ill health, dietary fats, essential fats, docosahexaenoic acid (DHA)
Final Published version and preferred citation
Crawford MA, Wang Y, Marsh DE, Johnson MR, Ogundipe E, Ibrahim A, Rajkumar H, Kowsalya S, Kothapalli KSD, Brenna JT. Neurodevelopment, nutrition and genetics. A contemporary retrospective on neurocognitive health on the occasion of the 100th anniversary of the National Institute of Nutrition, Hyderabad, India.
Prostaglandins Leukot Essent Fatty Acids. 2022 May;180:102427.
doi: 10.1016/j.plefa.2022.102427. Epub 2022 Apr 6. PMID: 35413515; PMCID: PMC9152880.
https://www.plefa.com/article/S0952-3278(22)00039-4/fulltext
Highlights
• Brain famine results from shortage of nutrients required for brain development.
• Brain disorders, seldom listed among NCDs, tops the global burden of disease.
• Enduring balanced supply of brain supportive foods should be a global priority.
• Equity and equality come together in properly nourishing mothers and babies.
Abstract
In celebration of the centenary of the National Institute of Nutrition (NIN), Hyderabad, India (1918-2018), a symposium highlighted the progress in nutrition knowledge made over the century, as well as major gaps in implementation of that knowledge. Brain famine caused by a shortage of nutrients required for perinatal brain development has unfortunately become a global reality, even as protein-calorie famine was largely averted by the development of high yield crops. While malnutrition remains widespread, the neglect of global food policies that support brain development and maintenance are most alarming. Brain disorders now top the list of the global burden of disease, even with obesity rising throughout the world. Neurocognitive health, remarkably, is seldom listed among the non-communicable diseases (NCDs) and is therefore seldom considered as a component of food policy. Most notably, the health of mothers before conception and through pregnancy as mediated by proper nutrition has been neglected by the current focus on early death in non-neurocognitive NCDs, thereby compromising intellectual development of the ensuing generations. Foods with balanced essential fatty acids and ample absorbable micronutrients are plentiful for populations with access to shore-based foods, but deficient only a few kilometres away from the sea. Sustained access to brain supportive foods is a priority for India and throughout the world to enable each child to develop to their intellectual potential, and support a prosperous, just, and peaceful world. Nutrition education and food policy should place the nutritional requirements for the brain on top of the list of priorities.
Introduction
Humans are the large land-based animal that evolved with an outsized brain. Human nutrition is special because of the remarkable demands of the big brain for building blocks and energy [1]. The dramatic increase in the World population over the last century is enabled by the stunning increase in calories and protein to grow and maintain brawn, with little concern to support brains. Food production emphasizing inexpensive commodities, especially calorie-rich grains and intensively produced animal foods, deliver altered nutrient profiles compared to nutrient-rich wild traditional foods. Manifestation of nutrient deficiency in the most complex and sensitive organ, the brain, is seen in the rise of mental ill-health and dropping world IQ [2, 3].
India’s National Institute of Nutrition (NIN) was founded by Sir Robert McCarrison in 1918 as ‘Beri-Beri’ Enquiry Unit in a single room laboratory at the Pasteur Institute, Coonoor, Tamil Nadu [4, 5]. McCarrison was a major-general and a doctor in the British Army. He had been deeply concerned at the health state of the population in the cities. When posted to Coonoor, a small settlement at 1,850 m above sea level in the South West of India, he was impressed by the health of the people. This led him to experimentally compare the diet of the people of Coonoor with those of the cities. The experiments conducted by Sir Robert McCarrison must rank as one of the most important studies in nutrition ever done [6, 7]. You could not repeat these experiments today because laboratory rats are genetically identical, to minimize variability for researcher. With the full complement of genetic variability in wild mice, he reproduced the variety of health disorders seen in the city population — not just one disease but a cluster thrown up by the individual-to-individual variation in genetic susceptibility [8]. This is what we see today. In Chennai, New Delhi, Kolkata, Mumbai or other Urban areas in India we see the double-edged morbidity from Western over-consumption, side by side with stunting and inadequacies of malnourishment [9-11].
When the NIN in India, was formed in 1918, end of world war one (WW I) the war to end all wars had come to its end. The pioneering work leading to the emergence of NIN in India demonstrated a truth for all time. Sir Robert McCarrison, the first director, and his co-workers, showed that a ‘bad’ diet resulted in not just one disease or disorder, but a cluster of them. The experimental and observational work concluded that a ‘good’ diet came from “the unsophisticated foods of Nature”. Departure from this diet led to disease and disorder [12].
To this day we do not have a better description of real balanced diet. The principles of essential nutrients were worked out in the 20th century focused on multigenerational survival in experimental animals, mostly rodents, that do not rely on higher order mental function [13]. The interplay of components of whole foods was lost with the success of the purified rodent diet intended to support life without frank deficiency but ignores health [14] and in this sense knowledge of nutrition is incomplete. At the same time, the food industry with the nominally laudable goal of providing inexpensive food, ignored brain health as agriculture intensified and the full measure of nutrients, many that reduce shelf life, were purged from the food supply. Modern dieticians recognize the concept of dietary patterns, meant to convey that it is the totality of the diet’s composition that determines well-being.
Human evolution started with the separation from the great apes some 5-7 million years ago. The human genome is only 1.24% different from that of the chimpanzee [15], highlighting that our physiology is adapted to wild foods, an uncompromising truth. In particular, the diet that brought us into being was one which drove encephalization from the modest 340g brain, of our closest relative, the chimpanzee, to the 1,450g of the Herto hominins at least 160,000 years ago [16, 17]. The conditions that led to the brain expansion must have included nutrients surfeit for the unique requirements of neural tissue.
An abundance of brain selective nutrients in the shore based diet [18] affected neural gene expression [19] and provided the structural lipids to support neural development of the expanding brain. Equipped with a balance of the best from land and sea the nutrition of the women would have led to epigenetic and genetic enhancement of brain size and function, generation after generation leading to humanity. Here we emphasize adequate dietary source of specific brain selective nutrients are needed for the proper maintenance of brain structure and function. The current notion of a balanced diet is based on present day practice. This is a weak notion. For a start, it does not care for the special needs of the brain. The evidence today is that our present diet fails the test. Mental ill-health has been escalating, and since the 1970s, measures of intelligence have been falling [3]. The dramatic rise in mental health issues in the recent COVID-19 pandemic reflects fragility rather than resilience. The message from the NIN has been lost and must be rediscovered.
Nutrition and Hope
The time of the formation of the NIN was a period of great discoveries in nutrition. The understanding of the function of essential amino acids and vitamins with molecular assessment and chemical synthesis as proof of function, unlocked a new, nutritional panorama for the conquest of malnutrition and its consequences in wasting and death or lifelong disabilities. The triumph over terminal, pernicious anemia with a dose of liver [20], the discovery of insulin [21] and other hormones raised hope to a new level where infectious and nutritional disorders would become past history. Barely 20 years later, that hope at the end of the 1914-18 WW I was lost.
However, World War Two (WW II) saw rapid advances in the public health policies and technologies. Nutritional advances also came from the public health perspective introduced by a professor of biochemistry — Jack Drummond who was commandeered to lead UK food policy. With support from Hugh Sinclair, Elsie Widdowson, Sir Robert McCarrison and others, he transformed policy to include universal education in the form of the Radio Doctor [22] and food policy with as close an approximation to Sir Robert’s unsophisticated foods of Nature. Every pregnant woman and those with infants and children, had cod liver oil, orange juice and milk delivered free to their front door by the Milk Man. When rationing came, sugar, confectionaries, ham, meat and butter were included. Fish and sea foods were never rationed. The objective was nutrition and health and it worked. The pre-war rise in death from heart disease faltered during the war. Misguidedly, Drummond’s policy was abandoned after the war. Policy changed to extensive production, food became debased (e.g. [23, 24]) and mortality began to rise again. Interventions eventually succeeded in reducing mortality, but we now have obesity, type 2 diabetes (T2D) leading to dementia and a return of cardiovascular mortality and stroke with decline in longevity. More worrying, the cost of mental ill-health has been rising, which we will return to later.
Genomic Centralism and Nutritional Minimalism
When WW II ended, hope was then re-ignited with the identification of the double helical structure of DNA and deciphering of the genetic code by Watson, Crick and Rosalind Franklin [25]. DNA with its four-letter code, led to an era of great excitement in the boundless hope of identifying a gene which can cause a particular disorder. Once identified it could be corrected.
The key to this particular hope was Crick’s central dogma which stated that once ‘information’ has passed into protein it cannot get out again. More colloquially it says that information goes from DNA to protein and never the reverse. The nature versus nurture argument was apparently settled. A health problem? Find the DNA code and all will be sorted, clearly proteins regulate DNA beyond just epigenetics.
But was Crick correct? Life began on this planet, about three and a half billion years ago or so. Time passed and after five sixths of the span of life, the oxygen tension rose to make air-breathing animal life thermodynamically possible. This climate change led to the Cambrian Explosion when all phyla known today came into existence [26]. That monumental event flatly contradicts the central dogma. Let us not belittle the significance of DNA. It is like decoding God’s Enigma Machine. At the same time money has poured into genomic-centred science, and it has arisen with little attention to the converse of the dogma: the reverse. The converse with the 1918 NIN message was swept aside.
In the modern era, the central dogma fell in part due to the work of Barbara McClintock establishing that "...the action of genes had to be and was controlled." [27] Information does flow from the external to DNA. In recent years molecular details of epigenetics have emerged. We now know environmental or nutritional conditions can alter the way DNA behaves without changing the basic genetic code [28], switching genes on or off. This has profound implications for health. It explains the rise in death from heart disease as a rarity in 1900 to become the no. 1 killer by 1960 [29], the striking contrast in disease pattern between Japan and the USA or East Africa and Europe. Much data now illustrates the effect of various nutrients on gene expression and of particular interest here is the effect of docosahexaenoic acid (DHA) on upregulation of genes in the brain which is apparent without changing membrane composition [19, 30]. Crick’s Dogma is odd because at that time it was already known that certain nutrients caused “enzyme induction”, i.e. a substrate could turn on synthesis [31]. Enzyme induction had to mean an influence on gene expression.
Epigenetics explains the situation in the Gulf States, which have risen from a seafaring and desert food culture to be, in just two generations, the world leader of T2D with the predictable follow-on of early dementia [32, 33]. It explains the globalization of the modern Western food system and with it, side by side, the Western disease pattern is now being seen in Beijing, Lanzhou, Harbin and Shanghai as well as Mumbai, New Delhi and Kolkata, not to mention Rio de Janeiro. These are but a few of the places around the world where supermarkets are full of Western, often subsidized foodstuffs, convenience foods. The sale of these products of intensive food production for cheap foods comes with the knowledge that it is changing cultures and exporting non- communicable disease. The moral right of expansion of such foods to basal commodities rather than occasional indulgences seems never to have been questioned. Interestingly, over a century and a half before, in 1859, Darwin had understood this fallacy. He had recognized two forces within his concept of ‘natural selection’: which was to be misinterpreted within a few years of his death in 1882.
The first of Darwin’s two forces consisted of the power of the ‘natural forces’ of the environment — sometimes known as ‘the impact energies’ … climate, temperature, light and dark, the quality of soil and nutrition - i.e. the chemistry and physics of the natural environment — thus ‘natural’: the second force being the ‘selection’ of those species which were ‘best-fitted’ to this matrix of the specific environmental conditions, as they, of course, would thrive best by adapting best, or through epigenetics, being adapted to their own specific conditions — thus ‘best-fitted’. Of the two forces, he wrote: the latter [the conditions] was the most powerful [34] (for clarification see the last paragraph of chapter 6 in all editions of the Origins of Species.)
However, Darwin's concern with the “conditions of existence”, was swept aside in the so-called ‘neo-Darwinism’ of August Weismann which removed all considerations for environmental factors [35]. Weismann cut the tails off nine generations of mice and when the following generations grew tails, declared that the conditions of existence had failed and claimed: “the all-sufficiency of natural selection” [36]. Darwin’s conditions of existence were considered too Lamarckian. The influence of Darwin’s conditions of existence — what he once termed pangenesis, is what is now collectively known as epigenetics [37].
This was a mistake which sadly has been perpetuated. Nutrition is the major environmental influence. As with Darwin’s full message, the founding message of the NIN was also lost.
Global Interest in the Brain is Lacking
The World Health Organization (WHO) has been concerned with infectious diseases as has the International Rotary Club with the aim of eradicating polio. At the same time heart disease and cancer was killing and maiming more people than HIV, malaria, measles, polio and tuberculosis combined. The world has changed in less than a century.
The WHO has now come up with a clarion call to deal with non-communicable diseases (NCDs). “The WHO Global Coordination Mechanism on prevention and control of NCDs (GCM/NCD) was established by the WHO Director-General on 15 September 2014. The scope and purpose of the GCM/NCD is to enhance the coordination of activities, multi-stakeholder engagement and action across sectors in order to contribute to the implementation of the WHO Global NCD Action Plan 2013-2020.” The WHO website explains [38]: “The 4 main types of non-communicable diseases are cardiovascular diseases (like heart attacks and stroke), cancers, chronic respiratory diseases (such as chronic obstructive pulmonary disease and asthma) and diabetes.”
The brain is missing from the list. This oversight is not surprising as despite the fact that it is the brain which makes us who we are, the brain is consistently omitted from health considerations. On the one hand, it is held in awe but on the other, taken for granted. Its disorders, most commonly, are mistakenly stigmatized. Mental problems are seen as a consequence of lack of parental care, abuse, or some form of disturbing experience in the person’s early years. In any of these cases, the individual is seen as a ‘sad case’ often to be blamed for failing to overcome the problem, whatever it was, or for a lack of moral fibre. The biological basis seems to have been ignored. A sickness of the skin, kidneys or liver can be readily identified as biological in origin despite having different signs. Sickness of the brain is somehow another matter. Fortunately, this attitude is now changing, and the biological origin of brain ill-health is now being recognized: not before time as mental ill-health is now escalating.
The steepest rise in mental ill health is in young children drawing in clinical attention to interventions [39]. In 1972 it was predicted that brain disorders would rise following on the rise in death from the heart disease [40]. The review of the book in the UK newspaper Sunday Times by Graham Rose (5th November 1972) made the gravity of the prediction clear. The prediction was based on
- (i) the evidence considered to exist between, dietary fats and cardio-vascular disease. Such evidence as there was referred only to non-essential fatty acids and neglected the positive role of essential fats, (ii) The dependence of brain development on prior vascular development, first in the placenta and then the fetus. The implication was that vascular disease would have to affect vascular performance in nourishing the fetus where brain development and growth is the priority, (iii) The similarity of nutritional requirements for specialized dietary fats for brain and vascular development and function. However, the brain cell development largely takes place before birth and is consequently better protected than the vascular system which continues to grow into adulthood. That prediction has been fulfilled with brain disorders having now overtaken all other burdens on ill health in the UK and in the 27-member states of the EU, and (iv) The dependence of the brain on a highly conserved profile of essential fatty acid derivatives which is significant to the story of evolution.
One in five adults in US are reported to live with mental illness in 2019, prior to the pandemic [41]; the number of 197·3 million in India suffer from mental disorders in 2017 is of the same order, double digit percentage of population [42].
It is worth noting that, in the 1970s the concept of maternal nutrition, low birth weight and adverse outcomes in learning and behavior were not new topics. A book by Birch and Gussow published in 1970 spelt out the risks [43]. Only a decade later saw the Black Report in the UK seeking action on low birthweight and pregnancy outcomes [44]. Lack of action saw another report by Sir Donald Acheson [45]. Today the prevalence of low birthweight and preterm birth in the UK, has if anything increased.
The European Union carried out an audit of the costs of health. The report published in 2005 claimed brain disorders to have overtaken the cost of other burdens of ill health [46]. In 2007, Dr Jo Nurse, a division head within the Department of Health (DoH) of the UK, estimated the cost of ill-health in the UK, and found that mental ill-health cost £77 billion in 2007: this was a cost greater than two of the WHO’s NCDs combined, namely cancer and heart disease [47].
Scepticism arose based on the claim that the high cost was due to new diagnostics. The EU re-assessed the cost in 2010 at 789 billion Euros. Dr Nurse did the same for the UK and found to be £105 billion in 2010. Independent analysis (Wellcome Trust web site) assessed the cost at £113 billion in 2013. Simon House, a trustee of the Mother and Child Foundation (UK), suggests these to be underestimates as violence was not included in the cost [48, 49]. Nor for that matter was cerebral palsy [48-50].
Earlier, Lord Rea of Eskdale (UK), co-coordinated and chaired a consultation on nutrition and mental health. They identified gaps in preventive research. The report refers to a Cochrane review by Lim et al in 2006 [51] which could not identify a single Randomized Clinical Trial (RCT) on ω3, in the dementias, despite the knowledge on their requirement for brain structure and function having been available since the 1970s. Lord Rea’s report recommended that “Because of the major potential benefit for the fields of education, crime, health and wellbeing of vulnerable sections of society, we believe more research is urgently needed in the area of nutrition and behaviour”.
It is astonishing, but hardly surprising given the history, that brain disorders are not in the WHO list of Non-Communicable Diseases. Make no mistake, the WHO is deeply concerned about mental ill-health and has a division devoted to it. But why this problem is not included in the NCDs is a mystery, although the stigma attached to disorders of the brain could be a relevant factor. Entailing the highest cost to health services and the community, it deserves to be up there with the other NCDs in the list of global priorities. Moreover, the fact that the prevalence of mental ill- health is increasing should be setting alarm bells ringing. If the primary organ which distinguishes us from the great apes is disintegrating, the future worldwide mental ill-health burden weighs heavily on societies and economies. That again is an elemental truth. One would have thought this was sufficient reason to put brain disorders at the top of the list. They should be included in WHO’s priority list of NCDs.
Eyewitness to Brain nutrition not a Part of Food Policy?
No government has a food and agricultural policy which prioritizes the brain. Most readers will be aware that protein is regarded as most important. Indeed, food policy is all to do with protein and calories. An all too typical declaration is striking because it was made at the world’s major food policy institute. At a meeting of the Food and Agricultural Organization of the United Nations, in its Headquarters in Rome, admittedly, in the early 1990s, the plight of the oceans was being discussed. A representative of the World Bank declared that the problem of the fish catches reaching its limit was not of real concern because people could get protein from other sources. That view reflects a common, dangerous misconception and a state of ignorance. David E Marsh attended meetings on the Foresight implications to the future food and agriculture. He raised the issue of the importance of the marine food web for brain lipids and the potential of farming the sea as is being done in Japan. On both occasions his comments were dismissed. Amongst other negative responses, it was claimed the oceans would anyway be polluted by 2034. The FDA in the USA has warned pregnant women to restrict fish and sea food intakes during pregnancy because of the threat of neurotoxicity from the mercury content. This, despite a paper describing significant benefits to verbal IQ, behavioural and social scores of children born to mothers who ate fish and sea foods well in excess of the 12 ounces (2 average meals) a week from the FDA recommendation [52]. Astonishingly, the FDA seemed not to have considered the Japanese data. Japanese mothers eat fish and sea food nearly every day and perhaps more than once a day. Where is the evidence of neurotoxicity? Indeed, contrary to the FDA advisory, those mothers gave birth to the children who have the best longevity, the least major depression, least heart disease and common cancers of the industrialized nations. The FDA may have had in mind fish caught in heavily polluted lakes and estuaries in the US which would be reason for not recommending to pregnant women. A recent systematic review styled according to the methods used by the U.S. Dietary Guidelines Advisory Committee (DGAC) revealed overwhelmingly positive effects on neurocognitive development of seafood consumption on children’s neurocognitive development, whether eaten by pregnant mothers or children themselves [53]. The DGAC report went even further in extoling the reliance on seafood to maintain health in pregnancy and through the life cycle, attributed at least in part to omega-3 fatty acids. The 2020 DGAC noted that many studies found negligible harm from seafood consumption despite having been designed to search for negative effects [54]. At this writing US food policy has not yet been adjusted to reflect this clear and convincing evidence.
As these examples show, for some reason the brain has mostly been side-lined. Brain composition contains as much structural fat as protein [55]: very special fats, marine fats into the bargain which you have to get from food or make with difficulty from green foods. Remember, the brain evolved in the sea some 500 million year ago and it was built with sea foods. There was nothing else.
Study of human milk is a good place to start. It has the least amount of protein of all large mammals but is stuffed with the essential fats needed to finalize brain development and growth. Seventy percent of the energy the mother devotes to fetal development is soaked up by the growth of the fetal brain. In the adult, the brain consumes 20% of the resting energy despite being only 2% of the body weight. Moreover, 26% of the energy, consumed by the brain is used for the maintenance of the membrane fatty acids [56]. Nature clearly declares the brain is the biological priority for Homo sapiens and does so before and after birth. Breast milk is rich in palmitate and other fats as fuel for energy and the essential fats needed to finalize neurodevelopment. Is it not about time that the needs for the brain are parachuted into the global and country wide assessment of human nutritional needs?
Food and the Population Crisis
Now we come to food security. In the 1960s, faced with a population of 3 billion and an expectation of 6 billion by 2000 many writers voiced concern over the population entering its logarithmic phase of growth with the expected failure of food resources to keep pace. Julian Huxley became the first Director General of UNESCO [57]. He wrote this:
“The world has to achieve the difficult task of reversing the direction of its thought about population. It has to begin thinking that our aim should be not increase but decrease — immediate decrease in the rate of population-growth; and in the long run, decrease in the absolute number of people in the world.”
The young Sir David Attenborough expressed concern: In 1976 he wrote:
“There are so many of us that the seas and skies are not big enough to cope with he over whelming quantities of waste and we are now choking in our own filth.” And “The yearly increase in population shows no sign of slackening, but it cannot continue for ever without ending in disaster” [58].
The Foresight Think Tank (UK) spent 2 years with 400 specialists compiling a report for governments on the ‘Future of Food and Agriculture’ [59]. The brain was mentioned en passant, but the discussion was all about protein and calories. The oceans were mentioned — only briefly. It is actually a brilliant report with critically important conclusions. It refers to the scarcity of new arable land to bring into production to meet the needs of the rising human population. It would have been so much stronger had it tackled the highly specific requirements for the brain because that is what makes us different from other animals.
Many other predictors of mass famine, speculating about Malthusian population growth outstripping resources, were wildly off target. The development of high yield grain varieties by agricultural science enabled many billions of greater population carrying capacity of the planet. In one sense however, mass famine did happen — brain famine — because of the emphasis on calories and protein. Calorie and protein production outstripped the production of brain-selective nutrients, leading to brain famine. The undisputed global malnutrition of iodine, iron, and vitamin A, so called “hidden hunger”, were an unmistakable bellwether that the job of feeding the brain was incomplete even decades ago. Arguments over the fundamental facts of nutrition and metabolism of omega-3 fatty acids as an irreplaceable building block of the brain, the brain’s fundamental infrastructure, continue to this day. Studies in the 1980s and 1990s show that maternal overnutrition with omega-6 linoleic acid antagonist to depleted omega-3 invariably lead to all manner of neurological abnormalities in the offspring, notably aggression, problems of impulse control, anxiety, depression, and impaired executive function [60]. More recent studies show that certain genotypes are likely more susceptible to these effects [61].
The Shrinking Brain.
Dr Marta Lahr from the UK Cambridge University's Leverhulme Centre for Human Evolutionary Studies presented her findings to the Royal Society in 2013 [62] that the brain size of the modern-day humans has been shrinking since the beginning of the land-based agriculture and animal husbandry 10,000 years ago. Scientific method is about prediction and test. If you know how the brain got here then you can predict what will happen in the future.
The UK newspaper Daily Mail of 11th January 2014 reported: “The boss of Morrisons, a major UK supermarket chain, has slammed Britain's educational system claiming many school leavers who turn up at his stores asking for jobs can't spell or add up.... The poor results come despite a near doubling of the education budget between 1997 and 2010 under Labour — from £50 billion a year to £89 billion”. This is not exactly a new story. Figure 1 shows falling world IQ levels over time [3].
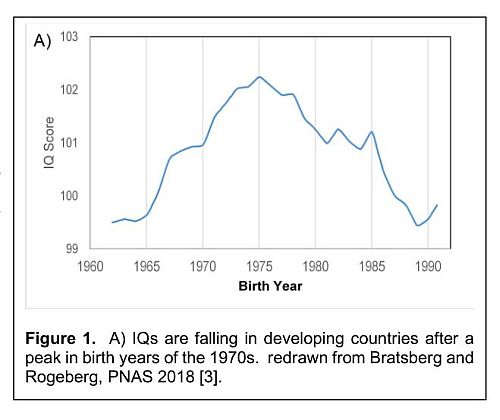
The Flynn effect is the incremental rise in IQ since Victorian times. However, the data on IQ varies. The above diagram is suggestive of a global reversal. This would be consistent with the change in food system and increased reliance on intensification of land-based foods (e.g1. [24, 63] since the abandonment of Sir Jack Drummond’s WWII policies and the now rise in T2D and obesity. For example, the amount of DHA in 100g chicken meat in 1970 was 170mg, and in 2002 it was 25mg. At the same time the amount of carcass fat had increased several fold. Intensive beef production produces 6.5 times the energy from carcass fat compared to protein. The trend can be reversed with attention to production: farmed salmon DHA can exceed DHA in wild salmon with careful attention to feeding DHA to the salmon, though even here the linoleic acid content of the fat is far higher than in the wild due to grain use [64].
After discussing the lack of global interest in brain health, brain nutrition not being a part of food policy and shrinking brain issues, now we connect to NIN (2018) with balanced diet and genetics.
NIN 2018 and the Balanced Diet.
The worlds of 1918 and 2018 are so very different. There are now 6 billion more mouths to feed than in 1918. Despite the notable advances in technology and science, 600,000 children died from malnutrition during 2018 and some two billion people are at risk of intellectual disability from iodine deficiency. Mental ill-health is now the costliest burden of ill-health. It is an important time for Governments led by organizations like the NIN, to address this issue. As brain development and mental health starts before the birth the primary solution has to be a health and nutrition policy for the mother. Indeed, one cannot stress too strongly the importance of the relevant education and nourishment of young girls, and for that matter the boys as well.
None of this will be of use unless governments ensure that the food and agricultural systems prioritize the needs of the brain. The brain evolved in the sea hence this starts with restoring our links with aquatic food resources to ensure adequate provision of DHA [65] and its accessory trace elements essential for the function of peroxidation defensive enzymes [66] like iodine, selenium [67, 68], zinc [69], copper [70], manganese [71] and B12 [72]. DHA is essential for the production, maintenance and function of photo-receptors [73], synapses [74] and neurons [65].
Again, we meet the problem with the balanced diet. The ω9, ω6 and ω3 fatty acid families use the same enzyme systems hence an imbalance causes one to be an antagonist of the others.
Too much LA will be detrimental to the ω3 status, specifically DHA [75, 76]. The production of LA rich seed oils is a new phenomenon which started in the 1950s with the idea of replacing saturated fats with polyunsaturated fats from a small component of the diet and has increased dramatically using LA rich vegetable oils [77]. Prior to that time, olive oil was the principle vegetable oil in Europe and had been for several millennia. Olive oil is rich in oleic acid with relatively small amounts of LA. Reducing the reliance on LA seed oils in the food web would boost the bioavailability of any dietary DHA and facilitate its biosynthesis. However, for biosynthesis to be useful there needs to be a source of the parent precursor, a-linolenic acid (ALA) to be available in the diet.
Foods rich in ALA are uncommon, removed from the industrial food supply because ALA-rich foods have shorter shelf-lives [78]. An ALA primary source would be dark green vegetables and some leguminous beans. In India, the use of mustard seed oil was an especially rich source of ALA. Sadly, this traditional oil has been replaced to a significant degree by LA rich seed oils [79]. LA rich seed oils swamp ALA from leaves and beans and cause omega-3 deficiency. This retrograde step should be reversed. The balance of ω6 to ω3 in the brain is about 2 to 1 globally although in some regions like the photoreceptor, there is very little ω6 as the sn-2 position of the phosphoglycerides is saturated with DHA.
Imbalance of ω6 to ω3.
An imbalance in ω6 to ω3 polyunsaturated (PUFA) has long been of concern as industrial oils replaced traditional oils first in the US [80] and later in India [81]. Usually, nutritional scientists express this as a high ratio of ω6 to ω3 which has increased from 2-4 to 1 prior for traditional oil and fat sources, to present levels from 10-20 to 1. This concern is well supported based on the substitution of, for instance, commodity sunflower oil with ω6 LA at 75% of fatty acids while ω3 ALA is at trace levels, replacing milkfat with ω6 LA with 2.5% LA and 0.65% ALA. However, detailed consideration of the endogenous physiological response to these oils shows that unnaturally high LA levels dramatically suppresses ω3 metabolism through high overall PUFA levels. These concepts are well established since the 1960s and have been repeatedly reproduced over the decades [81-85]. Further, animal studies conducted at NIN demonstrated the importance of the balance of ω6 and ω3 for the prevention of diet related chronic diseases [86-89]. The key factor is endogenous DHA because it is the final product of ω3 biosynthesis in most tissues and is most suppressed by ω6 LA [81]. A cross sectional study showed very low intake of omega-3 PUFA among 625 school going healthy boys and girls aged between 7 and 13 years from Hyderabad, India [90]. A recent randomized, triple blinded, controlled clinical trial showed addition of DHA to lower linoleic acid foods improved cognitive abilities of severe acute malnourished children in Malawi [91].
Figure 2 is a redrawn version of the most comprehensive single study of whole body DHA status as measured by plasma phospholipid (PL) DHA [81]. Rats were fed 54 different diets with LA and ALA that vary in total amount and in ratio of LA to ALA. Notably, all DHA must be synthesized from ALA via the conventional pathway. If plasma PL DHA were controlled by the ratio of LA to ALA, best fits through the curves would be horizontal lines represented by the dashed lines for each of the three LA to ALA ratios, 9.4 (lowest), 2.3, and 0.7 (highest DHA). Manifestly they are not. The least squares lines show a maximum DHA at total PUFA <4% of energy; at these levels the ratio does control the peak plasma PL DHA (Figure 2A). Importantly, at total PUFA >about 8%en, the lines converge and there is little relationship between ratio and plasma PL DHA. In summary, below about total PUFA, LA to ALA is a key factor, but at high PUFA levels plasma PL is low and not responsive to ratio.
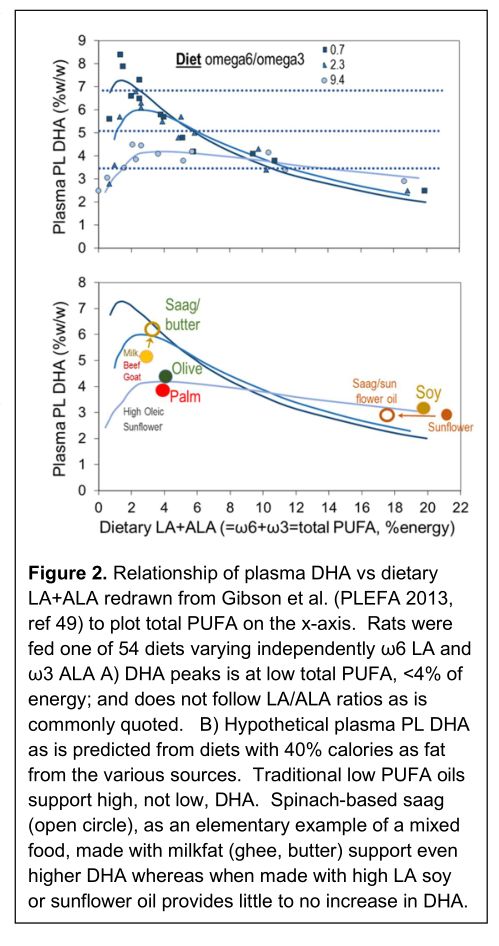
To explore this in the context of common edible oils and food, we replot the same least squares lines in Figure 2B, eliminating the actual data points for clarity. We instead plot the position of various edible oils eaten in a diet with 40% energy as fat with their LA and ALA contents at the plasma PL DHA level predicted by the curves. With industrial high ω6 seed oils typified by soy and sunflower, DHA synthesis is very low. However, with preindustrial fruit oils such as palm and olive, and animal fats from goat, beef, and milk, the predicted level of synthesis is greater because of the low PUFA content.
More significantly is the effect of addition of high ω3 ALA food to the oils, as in the preparation of a saag containing 450 g spinach and 450 g mustard greens naturally containing ALA, and 52 g butteroil (ghee). The LA to ALA ratio drops which increases the plasma PL DHA to near its maximum level. On the other hand, a saag made with soy or sunflower oil would only shift along the curves and no more plasma PL DHA would be found, reflecting the overwhelming amount of LA that inhibits DHA synthesis. The factor driving very low DHA synthesis is high intakes of LA in the background diets in all studies — abnormal compared to any known human diet prior to industrialization and the rise of high LA seed oils. LA is antagonistic with the last steps of DHA biosynthesis. Reduction of LA removes an effective block and circulating DHA rises. Key data are shown from our RCT [92]. These data collected in the Malawi show that a conventional Ready-to-Use Therapeutic Food (RUTF) high in LA causes a 25% drop in DHA over 4 weeks, while HO-RUTF high in oleic acid stabilizes DHA and causes a 60% increase in EPA.
Genetics.
Genes coding for the desaturation reactions, namely, fatty acid desaturase 1 (FADS1, OMIM#606148) and fatty acid desaturase 2 (FADS2, OMIM#606149) localize to the long arm of human chromosome 11 (11q12-q13.1) [93, 94]. FADS1 (ω5/ω7-desaturase, odd carbon chain desaturation) and FADS2 (ω6/ω8/ω4-desaturase, even carbon chain desaturation) have specificity for several fatty acid substrates [94-97]. The genetic variations within the genes mediating the endogenous synthesis of highly unsaturated fatty acids (HUFA) contribute widely to the variability and the efficiency of HUFA biosynthesis [98, 99]. The efficiency of HUFA biosynthesis most likely be controlled at multiple steps in the biosynthetic pathway either at the level of the desaturases (FADS2, FADS1) and/or the elongases (ELOVL2 and ELOVL5) depending on the genotype and the metabolic state [98, 100]. Several HUFA metabolism studies using stable isotope labeling, candidate gene single nucleotide polymorphisms (SNP), genome wide association studies (GWAS) and metabolomics showed interindividual variability in the conversion of precursor PUFA to HUFA products depends on genetic factors [98, 101-103]. A genetic component to the relative amounts of endogenous HUFA has been identified in recent work [104, 105]. DHA and its companion EPA generally oppose the action of the AA. The relative ratio of circulating DHA+EPA to AA has been linked to many chronic health conditions including cardiovascular disease [106] and depression [107]. We identified a specific functional genetic element, an insertion-deletion (Indel) polymorphic variant in the fatty acid desaturase (FADS) gene cluster that controls AA levels in widely divergent populations. Our recent data [105] show that genotype at this locus causes a dramatic different in equilibrium plasma PL AA. Using diet response curves for AA with high LA to ALA ratio (9.4) [81] similar to those for DHA, we plot a hypothetical relationship with dietary total PUFA (Figure 3) [61]. Between 0.5 and 3% of energy, the AA level rises rapidly and plateaus above about 3% of energy (Figure 3A). Using experimentally determined plateau levels, we show the difference in plateau levels for the three genotypes (Figure 3B) [61]. AA levels vary by an astonishing 57% depending on the genotype, with fast converters (I/I genotype) greater than slow converters (D/D genotype) and heterozygous (I/D genotype) converters in between (Figure 3C).
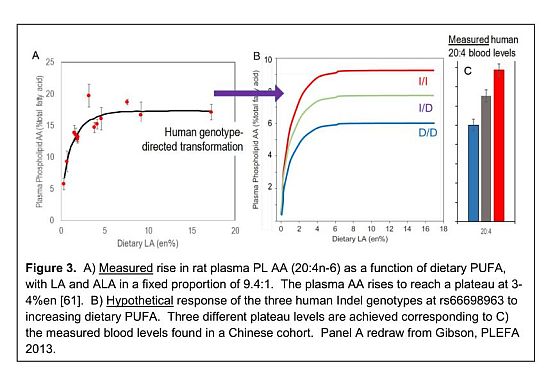
These observations have important implications for the dependence of HUFA milieu on a population basis. In a small sampling in the Pune region, we showed the population to be highly enriched in I/I individuals [104], likely reflecting their vegetarian heritage that requires more active synthesis of HUFA than seafood and meat consumers who would obtain HUFA in diet. When I/I fast converters consume diets with high LA and high PUFA oils, eg. sunflower and soy, their AA levels are maximal. Minor reductions in LA oils or addition of ALA has no effect on AA levels, and no effect on DHA levels because the enzymes needed to synthesize HUFA are well past the point of saturation [108]. However, traditional oils have lower PUFA and more moderate ratios, leading to less AA and more DHA. The next effect is that a vegetarian genetic heritage and high LA oils conspire to predispose to greater chronic disease through imbalanced HUFA. Moreover, the effect is of great concern for pregnant and lactating mothers and especially their children who rely on balanced HUFA to enable proper accretion of DHA as the brain expands in the first years of life.
Personalized HUFA Nutrition.
Commercially produced conventional seed oils, such as, sunflower, safflower, grapeseed, corn, and cottonseed are rich in ω6 LA. As ω6 and ω3 compete for the same enzymes in the HUFA biosynthetic pathway, individuals with the I/I genotype having fast metabolic capacity that supports high AA may be vulnerable to ill-health when adopting to a diet rich in ω6 LA which severely reduce synthesis of anti-inflammatory ω3 HUFA and increase synthesis of pro-inflammatory AA. Put it another way, diets high in ω6 LA may create a metabolic demand for ω3 EPA and DHA for I/I genotype and to a certain extent for I/D genotype. Balanced consumption of ω6 and ω3 precursors would be particularly important for I/I and I/D genotypes. The conventional ω6 LA rich seed oils dramatically reduces endogenous ability to synthesize and incorporate ω3 DHA and EPA in tissues, challenging the metabolic requirement for structural ω3 HUFA which is especially concentrated in neural tissue [100]. To support optimal neural development during pregnancy mothers selectively transfer DHA to fetuses. A recent Belgian cohort study showed imbalance of ω6 and ω3 HUFA was found to be associated with postpartum depression. Women in this study group having ω3 index <5% had a 5-fold increased risk of depressive episode [109].
We will come to another thorny issue later: the remainder of iodine deficiency in foods consumed by billions.
A Biomarker for Preterm Birth and Neurodevelopmental Disorder and Public Health.
Worldwide, highest 24% of all preterm births occur in India [110, 111]. One of the questions raised by Mulder et al [112] is DHA deficiency effective prenatally or after birth? In an RCT of a supplement containing DHA and arachidonic acid (AA) of 300 pregnant women, blood samples were taken at recruitment (ca. 12 weeks after conception), and at birth from mother and fetal cord blood. The total fatty acid profile of the red cell membrane was analyzed. The red cell membrane is a piece of maternal tissue. There are several variables which influence maternal status, and these include, diet, behaviour, absorption efficiency, genomics and metabolomics with the integration of all, defining what ends up in the membrane. Bill Lands once said “The tissue is the issue” and that applies here. The level of red cell membrane, oleic acid and other monounsaturated fatty acids at recruitment (ca. 12 weeks after conception) predicted preterm delivery at 34 and 30 weeks with a ROC of 0.926 and 0.852 respectively (p<0.000 and <0.017; Figure 4) [113].
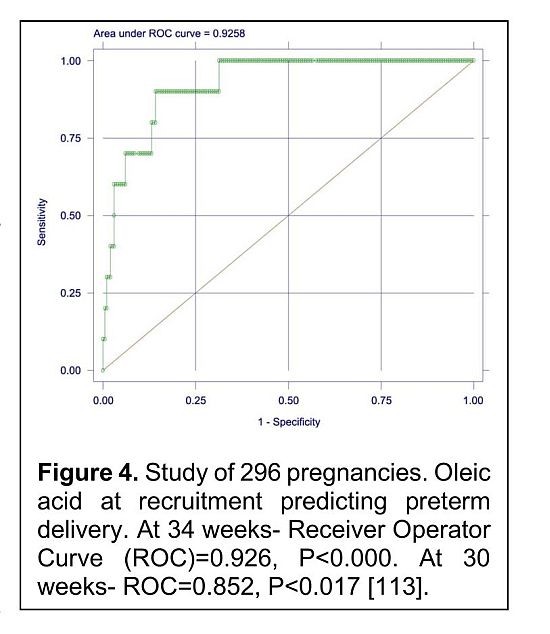
Membrane phospholipid oleic acid is known to rise in response to a deficit of AA and DHA which are key highly unsaturated fatty acids (HUFA) components of signalling, neuronal and glial cell membranes. Both are required substrates for the structure, growth and function of the brain [65, 114]. Deficiency results in the functional loss [115] i.e. this predictor is relevant to the high risk of neurodevelopmental disorders associated with premature birth and low birthweight. Different brain regions are built to different region-specific membrane lipid profiles [116, 117]. Moreover, brain lipid composition has been highly conserved through vertebrate evolution [114] and comparative evidence from 32 mammalian species indicates that a global improvision of the brain specific lipids is associated with a global loss of brain size [118].
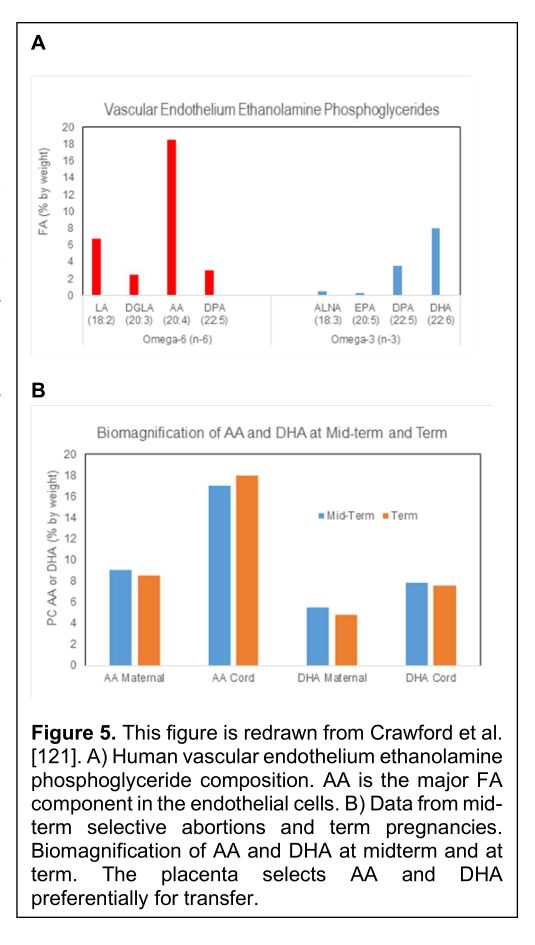
Oleic acid also identifies deficits in the fatty acids responsible for neural membrane growth and of eicosanoid regulators of immune and vascular function [119]. The proportion of oleic acid rises in the cell membrane lipids if the proportion of the HUFA falls for any reason. Preterm birth carries the highest risk for neurodevelopmental disorders. However, such disorders often have a vascular or immune background (Figure 5):
During the biomagnification across the placenta, LA is returned to the mother reducing the proportion in the cord blood choline phosphoglycerides into half.
The point here is that AA is being treated as the primary objective in placental fatty acid transfer. The likely explanation for prioritizing AA is that it is serving prenatal vascular and immune system development. It will also serve the glial cells. However, it is logical that the cardiovascular system has to come first in the order of organogenesis to provide the oxygen and nourishment.
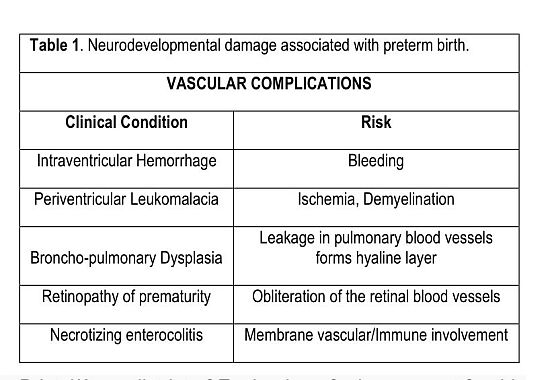
Intraventricular hemorrhage (prenatal bleeding) and periventricular leukomalacia (ischemia) is essentially a prenatal stroke. First this involves the vascular system then the immune response. If the ischemia scars there is permanent damage. DHA is effectively the only ω3 of consequence in prenatal vascular development. It could be involved in suppressing the inflammatory process which is a response to injury. Inadequate amounts in the locality could jeopardise injury resolution [120]. However, the role of AA as a major membrane constituent has not been properly evaluated. The likelihood is that it is important in membrane integrity and certainly responsible for prostacyclin synthesis which should keep the system flowing in good order. Indeed, the complications of prematurity have a vascular pathogenesis (Table 1) [121].
The DHA and ω3 eicosapentaenoic acid (EPA) deficiency in more than 60 studies of pregnant/lactating animals shows all manner of cognitive and mood disorders among the offspring, specifically cognitive, balance, and learning deficits, as well as higher aggression and poorer impulse control [60]. Emerging molecular and clinical evidence implicates ω3 deficits in heightened sensitivity to pain, where reduced diet ω6 and increased DHA and EPA reduces chronic headache from 10 hours to 4 hours per day, as well as associated psychological distress, likely by restoration of balanced signaling [122]. DHA and EPA both reduce neuroinflammation and cognitive decline [123]. DHA accumulates rapidly in the fetal brain starting at 6 months of pregnancy [124] and extending to age 18 when it stabilizes until at least age 90 at about 13% of FA [125], and >30% in synaptosomes. DHA is highly concentrated in grey matter and is influenced by the mix of dietary fatty acids perinatally during the brain growth spurt in humans and experimental primates [126-128]. Dietary DHA accelerates nerve development as measured directly by the progress of visual acuity in humans [129]. Retinal DHA, normally greater than 20%, is sensitive to diet. The intensity of the electrical response to light (the electroretinogram) depends on DHA content [130]. On the strength of this evidence, DHA is now included in nearly 100% of all infant formulas in the US. As calcium is to the bones, DHA is to the brain.
The Importance of Maternal Health and Nutrition before Conception.
The lifespan of red blood cell is around 120 days. Therefore, data obtained at about 12 weeks after conception, implies that the
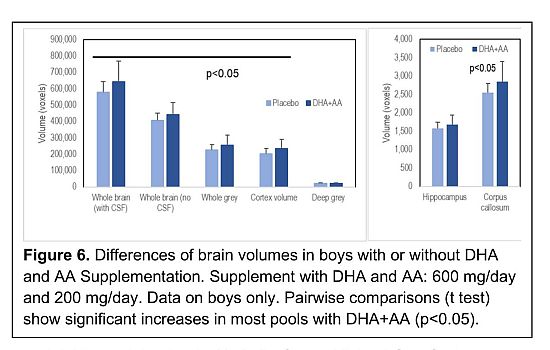
condition of the mother well before conception, determines the successful completion of pregnancy. Preterm birth carries the highest risk for neurodevelopmental disorders and potentially the neural development of the offspring. Indeed, high-quality MRI scans from our study, performed shortly after birth, showed that ω3-6 supplement promoted the growth of specific regions of the brain including the cortex (learning) and the corpus callosum (connectivity and possibly a key region involved in the development of autism) [131]. Further analysis of the placebo data, which is to be published, has revealed that the girls have a different response to the fatty acid status than the boys. This study opens the door to delineating the biological risks of different neurological disorders. As with neural tube defects, this and further studies could pave the way to prevention and possible therapies whilst the brain is still plastic.
The fact that we only saw a statistically significant enhancement of certain regions of the brain in the boys, could be a reflection of the heightened sensitivity of males to essential fatty acid deficiency/status which we observed was effective prenatally [132]. Although a much larger study and the inclusion of lipidomics in the analysis, might reveal an effect on the females, the effect such as it was, would have been unlikely to have had a major impact on neurogenesis. By 12 weeks into pregnancy, the cells that are to form the cortex are already migrating to do so. The lipidomic approach in relation to the differences in regional brain composition could be important to assess more detailed biomarker specificity in relation to specific neurodevelopmental disorders. Synaptogenesis and connectivity for example are relevant targets for this later stage of the neurodevelopment. However, as it stands, assessment of red cell membrane oleic acid could be used to reveal risk to premature birth and action then taken with current knowledge to prevent.
In considering the evident impact of food chemistry and environment, the composition of the membrane lipids responds to chemistry, temperature and pressure. Moreover, changes in lipids around the domains of membrane proteins change protein function.
This then leads to the concept of a new paradigm in biomedical science for this century linking environment, the genome and medicine in which lipidomics will be seen to play an, as yet, unheralded but major role in securing neurodevelopment [133].
A study of 11,875 pregnancies in the Bristol/Avon district of England, as further support for this approach. It described a linear increase in verbal IQ, and social behavioural scores measured in children at 8 years of age and dependent on the ω3 from fish and sea foods in the mother’s diet determined during pregnancy [52]. The mid pregnancy dietary data likely reflects the habitual diets and hence the diet of the mother leading up to conception.
We were able to obtain magnetic resonance images of 85 new-born brains to help test the effect of the supplementation (Figure 6). Despite being given early in pregnancy, it had no detectable effect in the girls but did enhance the whole brain and DHA rich regions including the cortex and the corpus callosum in the boys [131].
This MRI data limited as it is, provides additional evidence of the importance of lipid nutrition and through the relative weakness of the response adds strength to the concept that the condition of the mother when she conceives is of the greatest importance. Implantation takes place on day 7 after conception. That and subsequent events take place in the Milieu Intérieur [134] of the mother. Here we have another inescapable truth. Indeed, in terms of her fat stores and cell membrane composition, these will have been determined by her history. They constitute her Milieu Intérieur. Indeed, it is not far-fetched to say that the period of puberty could be relevant to the final condition and biological efficiency of the women to deliver a healthy newborn.
In 1976 Prof. R. W. Smithells and colleagues from the Department of Pediatrics and Child Health, Leeds, UK reported on the relationship between vitamin deficiency and neural tube defects. This report was followed up by an intervention study of multi vitamins which included folic acid as the main suspect for a deficiency cause [135]. In 1991 the Medical Research Council of the UK, published the results of an RCT with folic acid pre-conception providing convincing evidence of a causal relationship with folate deficiency [136]. Andrew Czeizel in Hungary has also drawn attention to the importance of the period around the time of conception and neurodevelopmental disorder. They assessed the impact of multi-vitamins prior to conception in 5,502 pregnancies.
Table 1. Neurodevelopmental damage associated with preterm birth. VASCULAR COMPLICATIONS Clinical Condition Risk Intraventricular Hemorrhage Bleeding Periventricular Leukomalacia Ischemia, Demyelination Broncho-pulmonary Dysplasia Leakage in pulmonary blood vessels forms hyaline layer Retinopathy of prematurity Obliteration of the retinal blood vessels Necrotizing enterocolitis Membrane vascular/Immune involvement
“Both intervention trials and observational studies confirmed that this new primary preventive method is effective - beyond the prevention of neural-tube defects - in the reduction of the most common structural birth defects: congenital cardiovascular abnormalities” [137, 138].
We are the first to admit that neurodevelopmental disorder is an ecological issue. It is not just one nutrient but the cluster of nutrients which together are required for optimal brain growth and development and for that matter for the full proper scope of organogenesis.
Coimbatore.
“Be the change you want to see in the World” Mahatma Gandhi.
The case for preconception care and the case for supporting neurodevelopment is incontrovertible. At the same time much can be done with education. Unfortunately, home economics was removed from the UK school curriculum starting in the 1960s, leaving us today
with leaders in governments and captains of industry, including the food industry, with little or no knowledge of nutrition and health. Hence, we have the consistent propagation of the protein myth [139].
“In India, 3,341,000 babies are born too soon, each year and 361,600 children under five die due to direct preterm complications” [140].
Following the NIN Diamond Jubilee celebration in 1978, Professor Michael Crawford went on a lecture tour in India at the courtesy of the British Council. One location was the Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore which was created in 1957 by Dr. T.S. Avinashilingam who funded and partnered Mahatma Gandhi in jail, turned out to be a true University. Dr. Rajammal. P. Devadas, an International Home Scientist and Nutritionist (IUNS Awardee, 2001, Austria) nurtured Avinashilingam University, India by empowering Women students towards Nutrition Education and Health Education for the Community. Its activities stretched out to work with the community and its schools. Its work was truly universal not just an ivory, academic tower. A most interesting part of the visit was to a school where the University staff shared teaching and extramural activities. The song which the children greeted the visitors was about the value of different foods (carrots being good for the eyes because ….).
The school children worked on the market garden where they milked the goats, fed the hens, collected eggs, took care of the chicks and tended the growing and harvesting of the vegetables. This activity combined with education in nutrition and home economics resulted in children entering the adult world with knowledge relevant to their health and well-being. Visits to the hospital suggested there was little malnutrition to be seen. In other regions, the doctors when asked said they were too busy and did not have time to engage in prevention.
The message of Coimbatore and the Avinashilingam University, India is a step in the needed order to face the nutrition challenge of today. Even now conducting a 10 day Community Nutrition Camps to assess the Nutritional Status of Population in a village is mandatory for Postgraduate students in Food Science and Nutrition and this adds value to their Education. Children were being empowered with knowledge about food and its meaning. Did not the management of the animals convey from an early age the principles of reproduction and the health of the new-born. And did that not also convey information on contraception? Whilst not every school by any means has the luxury of space for a market garden the message from Coimbatore clearly demonstrated the importance of education about food, nutrition and health which is sadly lacking even in medical colleges. The latter is all the more surprising when one considers that the bulk of the NCDs is related to nutrition and behaviour. Whilst infection is still not quite a feature of the past, nutrition and behaviour touches a very large part of the modern disorders and the daily work of the medical practitioner. For example, we have rampant obesity and T2D with its risk for heart disease, stroke and dementia. And is not nutritional science involved in the management of heart disease, renal and liver disease, cancer, and recovery from trauma and surgery, the management of pregnancy, depression and palliative care? That cluster requires more than just 3 hours of lectures on nutritional science in the training of our medicals. The Coimbatore experience and the interface between the College, the schools and the community, illustrated the power of education.
Iodine deficiency and the co-existence of ω3 Deficiency.
A topic of importance specifically to the neurodevelopment is iodine deficiency. There are some 2 billion people at risk for iodine deficiency worldwide. The richest food resource for iodine is in the marine food web. Iodine salts being water soluble, along with salts of other trace elements have been washed by millennia after millennia of melting snow and rain into the rivers and then into the sea. Within the large number at risk to iodine deficiency, pregnant women are especially vulnerable. Iodine deficiency during pregnancy is a sure way to cause intellectual disability and in extreme cases, cretinism in those born to iodine deficient mothers [141]. It is likely that iodine deficiency is associated with deficiencies of other trace elements and also a deficiency of DHA. Both lead to poor mental development with DHA deficiency a potential etiological agent for neuro-vascular developmental disorder [112, 142, 143].
In India, “About 167 million are estimated to be living in Iodine deficient endemic areas. Iodine deficiency causes goitre (enlargement of thyroid gland in the neck), neonatal hypothyroidism, cretinism among new born, intellectual disability, delayed motor development, stunting, deaf-mutism and neuromuscular disorders. The most important consequence of iodine deficiency in mothers is cretinism in which the children suffer from intellectual disability right from the birth. About 90,000 still-births and neonatal deaths occur every year due to maternal iodine deficiency. Around 54 million persons are estimated to have goitre, 2.2 million have cretinism and 6.6 million suffer from mild psycho-motor handicaps [144].”
Dr Coluthur Gopalan, FRS, organized a conference in New Delhi, on “The Brain” in 1998. Dr Gopalan was Former Director-General, Indian Council of Medical Research (ICMR), Former Director, National Institute of Nutrition, India and at the time was President, Nutrition Foundation of India. During the conference data was presented on iodine deficiency in Kerala where 60% of the school children had palpable goitre. However, the prevalence was only about 1% in those living in the coastal towns and villages. There was an almost identical situation in Indonesia 1990-1993, where Professor Michael Crawford and Dr Darwin Karyadi of the Ministry of Health in Bogor, conducted a survey which revealed that 60% of the school children had goitre. However, there were none in the fishing villages. A recent survey for goitre prevalence in India showed 263 out of 324 districts had IDD problem with a prevalence of more than 10% [145, 146]. There were about one million severely intellectual disability children and 800,000 cretins. Iodized salt seemed to be ineffective. The likely explanation was the high humidity and evaporative loss of the iodine combined with loss during cooking [147] as well as the use of goitrogenic foods which include cruciferous vegetables like broccoli, cabbage, cauliflower, horseradish, kale, mustard greens, radishes, and turnips and possibly other local foods.
The recommended solution for Indonesia was to grow kelp which is now being done enthusiastically off the coast of Bali. Kelp is rich in iodine, other trace elements. It also contains some ω3. It can be used directly as food, fertilizer to return iodine and trace elements to the soil and feed for animals. We are told the kelp farmers are making more money than the inland farmers. Regardless, the idea could be used in Kerala and indeed in other parts of India which has an extensive coastline.
There is another issue concerning iodine deficiency which is of concern. The richest source of iodine is in the marine food web which is also the richest source of DHA. The likelihood is that iodine deficiency is also a DHA deficiency.
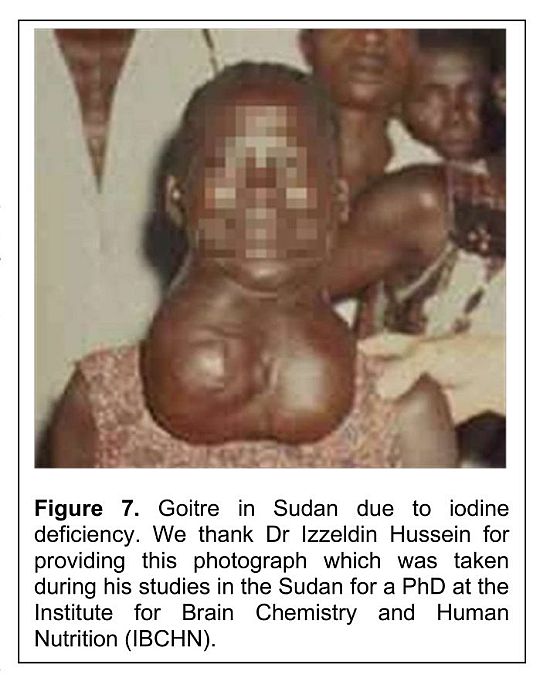
Support for this concern comes from our studies in the Sudan. Dr Izzeldin Hussein has described the presence of serious iodine deficiency in the Sudan [148]. The following illustration is one of many pictures he took to illustrate the severity of the condition (Figure 7).
At the same time Dr Kot Nyuar had been studying maternal nutrition and had analyzed milk composition as an index of essential fatty acid status [149]. Our laboratory has analyzed the composition of human milk in several thousand samples across the globe [150], including just over 2,000 for a WHO study in Thailand, Hungary and the UK [151]. We have never seen such low levels of milk DHA as encountered in the Sudan, which was 0.06% [149]. Similar levels of DHA (0.06%) were seen in milks from Pakistan [152]. These compare to a global mean of 0.32% DHA for studies available in 2007 [153].
The weakness of this conclusion is that the fatty acid and iodine data are not from the same individuals. Nonetheless, there is logic in the co-existence of iodine and DHA in the marine food web. With the paucity of DHA in the land-based food web, the coexistence of DHA and Iodine deficiency needs to be considered. Both deficiencies lead to deficits in brain function.
It is plausible that significant biosynthesis takes place in the Indian vegetarian population which may provide for brain development [154], though this capability is undoubtedly compromised when diets have high ω6 LA. However, the high prevalence of low birthweights and prematurity, coupled with iodine deficiency referred to in 2011 Dietary Guidelines of India, are a cause for concern. One can ask, how many people know the dietary sources for ALA? Do people spontaneously eat enough of these to provide for optimum neurogenesis in pregnancy? How much is enough?
We do not know if the Coimbatore school had a fishpond. However, the neighbouring China is the global leader in freshwater aquaculture. The Vietcong made fishponds out of large bomb craters [155]. The freshwater fish are reasonable sources of both DHA and AA, they will capture iodine with other trace elements and are generally good sources of protein and essential fatty acids. The development of coastal resources both for kelp, other sea foods and fish as is being done in Japan, China and Oman, would also help to correct the nutritional deficits and serve the priority of eliminating the high prevalence of low birthweight and prematurity with their high risk of mortality and neurodevelopmental issues and disorders.
We presented the importance of specific brain nutrients critical for the overall mental health with primary focus on the maternal and child health, perceived as a key issue in the Indian context. NIN (2022) has a bigger problem to solve- brain famine caused by a shortage of brain selective nutrients required for the overall brain health.
Conclusions
We lay out here some serious concerns with the rise in mental ill-health and population pressure on top of the list. These are important issues globally and particularly so in low income populations. We tried to present evidence on brain-specific nutrition and mental health which is especially relevant to malnutrition where it has been neglected. It is by no means all bad. There are so many good things in life. The world is full of love, music, literature, science, invention, history from which we can learn, the beauty of creation and art — good food and company. The cohesion of all these wonderful things is what makes us human. We have the knowledge and power to advance all of these in peace. In particular, to reverse the slide of intelligence and work towards achieving better mental and physical health for children. It is simply a matter of understanding the threat and setting the right priorities.
Acknowledgements.
We express our appreciation to DSM, Mead Johnson Nutrition and ICMR, Government of India who made the meeting in Hyderabad possible by providing unrestricted educational grants. We are grateful to the Mother and Child Foundation which funded the original work on maternal nutrition in the East-end of London, the student’s work in the Sudan and the RCT and MRI studies at Imperial College to which the Letten Foundation also contributed. Further support came from the Waterloo Foundation, Borne at Chelsea and Westminster Hospital, and Wellohi, China and Suntory, Japan. NIH grant R01 AT007003 from the National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements supported original work of JTB and KSDK. We are grateful to ICMR Director General Dr. Balram Bhargava for opening the symposium and delivering introductory remarks. All the authors have no conflicts of interest to declare.
Author Contributions.
MAC, KSDK and JTB: conception and design of the review. MAC, KSDK and JTB: wrote the first draft. All authors: critical revision, editing and approved it for the publication.
Conflict of Interest.
All authors declare that the present review paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] S.C. Cunnane, M.A. Crawford, Survival of the fattest: fat babies were the key to evolution of the large human brain, Comp Biochem Physiol A Mol Integr Physiol, 136 (2003) 17-26.
[2] R.V. Gow, J.R. Hibbeln, Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors, Child Adolesc Psychiatr Clin N Am, 23 (2014) 555-590.
[3] B. Bratsberg, O. Rogeberg, Flynn effect and its reversal are both environmentally caused, Proc Natl Acad Sci U S A, 115 (2018) 6674-6678.
[4] I.C.o.M.R. National Institute of Nutrition, India, https://www.nin.res.in/aboutus.html, (1918).
[5] A. Malhotra, Cutting Edge Research in the Contact Zone? The Establishment of the Nutritional Research Laboratories in Coonoor (1925-27), South Asia: Journal of South Asian Studies, 44 (2021) 117-134.
[6] A.R. Walker, The assessment and remedying of inadequate diets in India, as appreciated by Sir Robert McCarrison, Nutrition, 18 (2002) 106-109.
[7] R. McCarrison, A Lecture on SOME SURGICAL ASPECTS OF FAULTY NUTRITION, Br Med J, 1 (1931) 966-971.
[8] R. McCarrison, NUTRITION AND NATIONAL HEALTH: Lecture III. , Journal of the Royal Society of Arts, 84(4373) (1936) 1087-1107.
[9] A.J. Prendergast, J.H. Humphrey, The stunting syndrome in developing countries, Paediatr Int Child Health, 34 (2014) 250-265.
[10] J. Gouda, R.K. Prusty, Overweight and obesity among women by economic stratum in urban India, J Health Popul Nutr, 32 (2014) 79-88.
[11] P. Chatterjee, India sees parallel rise in malnutrition and obesity, Lancet, 360 (2002) 1948.
[12] R. Mccarrison, Nutrition And National Health. Cantor Lectures By Sir Robert Mccarrison, The British Medical Journal, Vol. 1, No. 3921 (1936) 427-430.
[13] C.J. Lowe, J.B. Morton, A.C. Reichelt, Adolescent obesity and dietary decision making a brain-health perspective, Lancet Child Adolesc Health, 4 (2020) 388-396.
[14] N. Daniel, L. Rossi Perazza, T.V. Varin, J. Trottier, B. Marcotte, P. St-Pierre, O. Barbier, B. Chassaing, A. Marette, Dietary fat and low fiber in purified diets differently impact the gut-liver
axis to promote obesity-linked metabolic impairments, Am J Physiol Gastrointest Liver Physiol, 320 (2021) G1014-G1033.
[15] I. Ebersberger, D. Metzler, C. Schwarz, S. Paabo, Genomewide comparison of DNA sequences between humans and chimpanzees, Am J Hum Genet, 70 (2002) 1490-1497.
[16] G.P. Rightmire, Out of Africa: modern human origins special feature: middle and later Pleistocene hominins in Africa and Southwest Asia, Proc Natl Acad Sci U S A, 106 (2009) 16046-16050.
[17] J.G. Herndon, J. Tigges, D.C. Anderson, S.A. Klumpp, H.M. McClure, Brain weight throughout the life span of the chimpanzee, J Comp Neurol, 409 (1999) 567-572.
[18] C.L. Broadhurst, Y. Wang, M.A. Crawford, S.C. Cunnane, J.E. Parkington, W.F. Schmidt, Brain-specific lipids from marine, lacustrine, or terrestrial food resources: potential impact on early African Homo sapiens, Comp Biochem Physiol B Biochem Mol Biol, 131 (2002) 653-673.
[19] K. Kitajka, A.J. Sinclair, R.S. Weisinger, H.S. Weisinger, M. Mathai, A.P. Jayasooriya, J.E. Halver, L.G. Puskas, Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression, Proc Natl Acad Sci U S A, 101 (2004) 10931-10936.
[20] G.R. Minot, W.P. Murphy, Treatment of pernicious anemia by a special diet. 1926, Yale J Biol Med, 74 (2001) 341-353.
[21] F.G. Banting, C.H. Best, J.B. Collip, W.R. Campbell, A.A. Fletcher, Pancreatic Extracts in the Treatment of Diabetes Mellitus, Can Med Assoc J, 12 (1922) 141-146.
[22] H.o.t.B.D.C.H.-T.R. Doctor, https://www.bbc.co.uk/programmes/p02f852d, (2014). 24
[23] M.A. Crawford, K. Ghebremeskel, The equation between food production, nutrition and health, In Food Ethics, ed Ben Mepham, Routeledge, London. , (1996) 64-100.
[24] Y. Wang, C. Lehane, K. Ghebremeskel, M.A. Crawford, Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein, Public Health Nutr, 13 (2010) 400-408.
[25] J.D. Watson, F.H. Crick, Genetical implications of the structure of deoxyribonucleic acid, Nature, 171 (1953) 964-967.
[26] D.E. Briggs, The Cambrian explosion, Curr Biol, 25 (2015) R864-868.
[27] U.S.N.L.o. Medicine., The Barbara McClintock Papers., https://profiles.nlm.nih.gov/spotlight/ll/feature/nobel, (2021).
[28] S.H. House, Epigenetics in adaptive evolution and development: the interplay between evolving species and epigenetic mechanisms: extract from Trygve Tollefsbol (ed.) (2011) Handbook of epigenetics--the new molecular and medical genetics. Chapter 26. Amsterdam, USA: Elsevier, pp. 423-446, Nutr Health, 22 (2013) 105-131.
[29] J.E. Dalen, J.S. Alpert, R.J. Goldberg, R.S. Weinstein, The epidemic of the 20(th) century: coronary heart disease, Am J Med, 127 (2014) 807-812.
[30] K.S. Kothapalli, J.C. Anthony, B.S. Pan, A.T. Hsieh, P.W. Nathanielsz, J.T. Brenna, Differential cerebral cortex transcriptomes of baboon neonates consuming moderate and high docosahexaenoic acid formulas, PLoS One, 2 (2007) e370.
[31] M. Dixon, E.C. Webb, Enzymes, Academic Press, eBook ISBN: 9781483225609, 2nd Edition (1964) 1-971.
[32] A.B. Khalil, S.A. Beshyah, N. Abdella, B. Afandi, M.M. Al-Arouj, F. Al-Awadi, M. Benbarka, A. Ben Nakhi, T.M. Fiad, A. Al Futaisi, A.A. Hassoun, W. Hussein, G. Kaddaha, I. Ksseiry, M. Al Lamki, A.A. Madani, F.A. Saber, Z. Abdel Aal, B. Morcos, H. Saadi, Diabesity in the Arabian Gulf: Challenges and Opportunities, Oman Med J, 33 (2018) 273-282.
[33] J.E. Hickey, S. Pryjmachuk, H. Waterman, Mental illness research in the Gulf Cooperation Council: a scoping review, Health Res Policy Syst, 14 (2016) 59.
[34] D.E. Marsh, The origins of diversity: Darwin's conditions and epigenetic variations, Nutr Health, 19 (2007) 103-132.
[35] Y. Zou, The Germ-Plasm: a Theory of Heredity (1893), by August Weismann". Embryo Project Encyclopedia, http://embryo.asu.edu/handle/10776/8284., (2015).
[36] D.E. Marsh, Darwin's passionate environmentalism or the dangerous fallacy of the 'All- sufficiency of natural selection' theory, Nutr Health, 21 (2012) 76-90.
[37] E.T. Tollefsbol, Handbook of Epigenetics: The New Molecular and Medical Genetics, Second Edition, eBook ISBN: 9780128054772, (2017).
[38] W.H. Organization, https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable- diseases, (2018).
[39] D.A. Moore, M. Nunns, L. Shaw, M. Rogers, E. Walker, T. Ford, R. Garside, O. Ukoumunne, P. Titman, R. Shafran, I. Heyman, R. Anderson, C. Dickens, R. Viner, S. Bennett, S. Logan, F. Lockhart, J. Thompson Coon, Interventions to improve the mental health of children and young people with long-term physical conditions: linked evidence syntheses, Health Technol Assess, 23 (2019) 1-164.
[40] M.A. Crawford, S. Crawford, What We Eat Today, Crawford MA, & SM (1972), Neville Spearman, SBN 85425 360 7, page 142, para 3, (1972).
[41] N.I.o. Health, https://www.nimh.nih.gov/health/statistics/mental-illness.shtml, (2021).
[42] C. India State-Level Disease Burden Initiative Mental Disorders, The burden of mental disorders across the states of India: the Global Burden of Disease Study 1990-2017, Lancet Psychiatry, 7 (2020) 148-161.
[43] H.G. Birch, J.D. Gussow, Disadvantaged children: health, nutrition and school failure., New York, USA, Harcourt, Brace & World, Inc.; Grune & Stratton, Inc., (1970).
[44] D. Black, J. Morris, C. Smith, P. Townsend, Inequalities in health. Report of a research working group, Health Visit, 53 (1980) 458.
[45] S.D. Acheson., "Our Healthier Nation; a Contract for Health" 5 February 1998, HMSO. Securing Our Future Health: Taking a Long-Term View., (1998).
[46] P. Andlin-Sobocki, B. Jonsson, H.U. Wittchen, J. Olesen, Cost of disorders of the brain in Europe, Eur J Neurol, 12 Suppl 1 (2005) 1-27.
[47] J. Nurse, Dr. Jo Nurse (DoH) at the Westminster Health Forum Keynote Seminar 17th July 2008., (2008).
[48] S. House, The Mother and Child Foundation - Prenatal nutrition: of paramount importance., https://www.fabresearch.org/viewItem.php?id=12320, (2018).
[49] S.H. House, Transgenerational healing: Educating children in genesis of healthy children, with focus on nutrition, emotion, and epigenetic effects on brain development, Nutr Health, 22 (2013) 9-45.
[50] S.H. House, Preconception Prevention of Brain & Behaviour Problems by Simon H House in: Sven Hildebrandt / Johanna Schacht / Alin Cotiga (eds.). , Obstetrics in Transition: Traumatic birth experience as a lifelong stress factor -healthy birth experience as a lifelong resource.
(2021), kt., ISBN 978-3-86809-167-0 (2021).
[51] W.S. Lim, J.K. Gammack, J. Van Niekerk, A.D. Dangour, Omega 3 fatty acid for the prevention of dementia, Cochrane Database Syst Rev, (2006) CD005379.
[52] J.R. Hibbeln, J.M. Davis, C. Steer, P. Emmett, I. Rogers, C. Williams, J. Golding, Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study, Lancet, 369 (2007) 578-585.
[53] J.R. Hibbeln, P. Spiller, J.T. Brenna, J. Golding, B.J. Holub, W.S. Harris, P. Kris-Etherton, B. Lands, S.L. Connor, G. Myers, J.J. Strain, M.A. Crawford, S.E. Carlson, Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews, Prostaglandins Leukot Essent Fatty Acids, 151 (2019) 14-36.
[54] D.G.A. Committee., Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. U.S. Department of Agriculture, Agricultural Research Service, Washington, DC., https://www.dietaryguidelines.gov/2020-advisory-committee-report (2020).
[55] M.A. Crawford, C.L. Broadhurst, The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: the challenge for human sustainability, Nutr Health, 21 (2012) 17-39.
[56] A.D. Purdon, S. Rapoport, Neural Energy Utilization: Handbook of Neurochemistry and Molecular Biology, (G, Gibsson and G. Dienelm eds,) Springer, New York. , (2007) 401-427.
[57] J. Huxley, UNESCO. General Conference, 1st, 1946 [242], Paris, France., https://unesdoc.unesco.org/search/N-EXPLORE-da50538e-9952-44d0-a981-6ccc383407a3, (1946).
[58] D. Attenburgh, Foreword to “Earth in Danger” by Ian Breach and Michael Crawford, Aldus Books, Doubleday ang Co,, NY, sbn: 385-11345-5, (1976).
[59] U. The Government Office for Science, Foresight: The Future of Food and Farming (2011) Final Project Report, The Government Office for Science, London, UK., (2011).
[60] J.T. Brenna, Animal studies of the functional consequences of suboptimal polyunsaturated fatty acid status during pregnancy, lactation and early post-natal life, Matern Child Nutr, 7 Suppl 2 (2011) 59-79.
[61] K.S.D. Kothapalli, H.G. Park, J.T. Brenna, Polyunsaturated fatty acid biosynthesis pathway and genetics. implications for interindividual variability in prothrombotic, inflammatory conditions such as COVID-19(,, bigstar, bigstar bigstar), Prostaglandins Leukot Essent Fatty Acids, 162 (2020) 102183.
[62] M. Lahr, https://phys.org/news/2011-06-farming-blame-size-brains.html#jCp, (2011).
[63] M. Crawford, K. Ghebremeskel, The equation between food production, nutrition and health. By Michael Crawford and Keb Ghebremeskel, In Food Ethics, ed Ben Mepham, Routeledge, London. 1996, pp64-100., (1996).
[64] D.P. Cladis, A.C. Kleiner, H.H. Freiser, C.R. Santerre, Fatty acid profiles of commercially available finfish fillets in the United States, Lipids, 49 (2014) 1005-1018.
[65] M.A. Crawford, C.L. Broadhurst, M. Guest, A. Nagar, Y. Wang, K. Ghebremeskel, W.F. Schmidt, A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution, Prostaglandins Leukot Essent Fatty Acids, 88 (2013) 5-13.
[66] K. Pong, Oxidative stress in neurodegenerative diseases: therapeutic implications for superoxide dismutase mimetics, Expert Opin Biol Ther, 3 (2003) 127-139.
[67] R. Brigelius-Flohe, L. Flohe, Selenium and redox signaling, Arch Biochem Biophys, 617 (2017) 48-59.
[68] N.D. Solovyev, Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling, J Inorg Biochem, 153 (2015) 1-12.
[69] S.D. Gower-Winter, C.W. Levenson, Zinc in the central nervous system: From molecules to behavior, Biofactors, 38 (2012) 186-193.
[70] I.F. Scheiber, J.F. Mercer, R. Dringen, Metabolism and functions of copper in brain, Prog Neurobiol, 116 (2014) 33-57.
[71] G. Bresciani, I.B. da Cruz, J. Gonzalez-Gallego, Manganese superoxide dismutase and oxidative stress modulation, Adv Clin Chem, 68 (2015) 87-130.
[72] E.E. van de Lagemaat, L. de Groot, E. van den Heuvel, Vitamin B12 in Relation to Oxidative Stress: A Systematic Review, Nutrients, 11 (2019).
[73] E.B. Rodriguez de Turco, N. Parkins, A.V. Ershov, N.G. Bazan, Selective retinal pigment epithelial cell lipid metabolism and remodeling conserves photoreceptor docosahexaenoic acid following phagocytosis, J Neurosci Res, 57 (1999) 479-486.
[74] H. Suzuki, S. Manabe, O. Wada, M.A. Crawford, Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice, Int J Vitam Nutr Res, 67 (1997) 272-278.
[75] A.Y. Taha, Y. Cheon, K.F. Faurot, B. Macintosh, S.F. Majchrzak-Hong, J.D. Mann, J.R. Hibbeln, A. Ringel, C.E. Ramsden, Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools, Prostaglandins Leukot Essent Fatty Acids, 90 (2014) 151-157.
[76] P. Budowski, M.A. Crawford, a-Linolenic acid as a regulator of the metabolism of arachidonic acid: dietary implications of the ratio, n-6:n-3 fatty acids, Proc Nutr Soc, 44 (1985) 221-229.
[77] T.L. Blasbalg, J.R. Hibbeln, C.E. Ramsden, S.F. Majchrzak, R.R. Rawlings, Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century, Am J Clin Nutr, 93 (2011) 950-962.
[78] A.H. Stark, R. Reifen, M.A. Crawford, Past and Present Insights on Alpha-linolenic Acid and the Omega-3 Fatty Acid Family, Crit Rev Food Sci Nutr, 56 (2016) 2261-2267.
[79] Ghafoorunissa, A. Vani, R. Laxmi, B. Sesikeran, Effects of dietary alpha-linolenic acid from blended oils on biochemical indices of coronary heart disease in Indians, Lipids, 37 (2002) 1077-1086.
[80] R.T. Holman, S.B. Johnson, P.L. Ogburn, Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation, Proc Natl Acad Sci U S A, 88 (1991) 4835-4839.
[81] R.A. Gibson, M.A. Neumann, E.L. Lien, K.A. Boyd, W.C. Tu, Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids, Prostaglandins Leukot Essent Fatty Acids, 88 (2013) 139-146.
[82] H. Mohrhauer, R.T. Holman, Effect of Linolenic Acid Upon the Metabolism of Linoleic Acid, J Nutr, 81 (1963) 67-74.
[83] H. Mohrhauer, R.T. Holman, Alteration of the Fatty Acid Composition of Brain Lipids by Varying Levels of Dietary Essential Fatty Acids, J Neurochem, 10 (1963) 523-530.
[84] R.T. Holman, The slow discovery of the importance of omega 3 essential fatty acids in human health, J Nutr, 128 (1998) 427S-433S.
[85] W.E. Lands, A. Morris, B. Libelt, Quantitative effects of dietary polyunsaturated fats on the composition of fatty acids in rat tissues, Lipids, 25 (1990) 505-516.
[86] Ghafoorunissa, A. Ibrahim, S. Natarajan, Substituting dietary linoleic acid with alpha- linolenic acid improves insulin sensitivity in sucrose fed rats, Biochim Biophys Acta, 1733 (2005) 67-75.
[87] A. Tyagi, U. Kumar, S. Reddy, V.S. Santosh, S.B. Mohammed, N.Z. Ehtesham, A. Ibrahim, Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with alpha- linolenic acid in a rat model of inflammatory bowel disease, Br J Nutr, 108 (2012) 1612-1622.
[88] A. Tyagi, U. Kumar, V.S. Santosh, S. Reddy, S.B. Mohammed, A. Ibrahim, Partial replacement of dietary linoleic acid with long chain n-3 polyunsaturated fatty acids protects against dextran sulfate sodium-induced colitis in rats, Prostaglandins Leukot Essent Fatty Acids, 91 (2014) 289-297.
[89] S. Jeyapal, S.R. Kona, S.V. Mullapudi, U.K. Putcha, P. Gurumurthy, A. Ibrahim, Substitution of linoleic acid with alpha-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis, Sci Rep, 8 (2018) 10953.
[90] D. Parasannanavar, I. Gaddam, T. Bukya, S.A. Ibrahim, K.S. Reddy, S.K. Banjara, B.P.P. Salvadi, B.N. Kumar, S.F. Rao, J.J.B. Geddam, H. Rajkumar, Omega-3 polyunsaturated fatty acid intake and plasma fatty acids of school going Indian children - a cross-sectional study, Prostaglandins Leukot Essent Fatty Acids, 170 (2021) 102294.
[91] K. Stephenson, M. Callaghan-Gillespie, K. Maleta, M. Nkhoma, M. George, H.G. Park, R. Lee, I. Humpheries-Cuff, R.J.S. Lacombe, D.R. Wegner, R.L. Canfield, J.T. Brenna, M.J. Manary, Low linoleic acid foods with added DHA given to Malawian children with severe acute malnutrition improve cognition: a randomized, triple blinded, controlled clinical trial, Am J Clin Nutr, (2021).
[92] J.C. Hsieh, L. Liu, M. Zeilani, S. Ickes, I. Trehan, K. Maleta, C. Craig, C. Thakwalakwa, L. Singh, J.T. Brenna, M.J. Manary, High-Oleic Ready-to-Use Therapeutic Food Maintains Docosahexaenoic Acid Status in Severe Malnutrition, Journal of pediatric gastroenterology and nutrition, 61 (2015) 138-143.
[93] A. Marquardt, H. Stohr, K. White, B.H. Weber, cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family, Genomics, 66 (2000) 175-183.
[94] H.G. Park, W.J. Park, K.S. Kothapalli, J.T. Brenna, The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells, FASEB J, 29 (2015) 3911-3919.
[95] W.J. Park, K.S. Kothapalli, P. Lawrence, C. Tyburczy, J.T. Brenna, An alternate pathway to long-chain polyunsaturates: the FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3, J Lipid Res, 50 (2009) 1195-1202.
[96] W.J. Park, K.S. Kothapalli, H.T. Reardon, P. Lawrence, S.B. Qian, J.T. Brenna, A novel FADS1 isoform potentiates FADS2-mediated production of eicosanoid precursor fatty acids, J Lipid Res, 53 (2012) 1502-1512.
[97] H.G. Park, M.G. Engel, K. Vogt-Lowell, P. Lawrence, K.S. Kothapalli, J.T. Brenna, The role of fatty acid desaturase (FADS) genes in oleic acid metabolism: FADS1 Delta7 desaturates 11- 20:1 to 7,11-20:2, Prostaglandins Leukot Essent Fatty Acids, 128 (2018) 21-25.
[98] J.Y. Zhang, K.S. Kothapalli, J.T. Brenna, Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis, Curr Opin Clin Nutr Metab Care, 19 (2016) 103-110.
[99] E. Lattka, T. Illig, J. Heinrich, B. Koletzko, Do FADS genotypes enhance our knowledge about fatty acid related phenotypes?, Clin Nutr, 29 (2010) 277-287.
[100] J.T. Brenna, Episodic Dietary DHA for Support of Tissue DHA, J Nutr, 149 (2019) 547- 548.
[101] J. Adamski, Genome-wide association studies with metabolomics, Genome Med, 4 (2012) 34.
[102] L. Schaeffer, H. Gohlke, M. Muller, I.M. Heid, L.J. Palmer, I. Kompauer, H. Demmelmair, T. Illig, B. Koletzko, J. Heinrich, Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids, Hum Mol Genet, 15 (2006) 1745-1756.
[103] E.A. Emken, R.O. Adlof, R.M. Gulley, Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males, Biochim Biophys Acta, 1213 (1994) 277-288.
[104] K.S. Kothapalli, K. Ye, M.S. Gadgil, S.E. Carlson, K.O. O'Brien, J.Y. Zhang, H.G. Park, K. Ojukwu, J. Zou, S.S. Hyon, K.S. Joshi, Z. Gu, A. Keinan, J.T. Brenna, Positive Selection on a Regulatory Insertion-Deletion Polymorphism in FADS2 Influences Apparent Endogenous Synthesis of Arachidonic Acid, Mol Biol Evol, 33 (2016) 1726-1739.
[105] P. Li, J. Zhao, K.S.D. Kothapalli, X. Li, H. Li, Y. Han, S. Mi, W. Zhao, Q. Li, H. Zhang, Y. Song, J.T. Brenna, Y. Gao, A regulatory insertion-deletion polymorphism in the FADS gene cluster influences PUFA and lipid profiles among Chinese adults: a population-based study, Am J Clin Nutr, 107 (2018) 867-875.
[106] J.E. Manson, N.R. Cook, I.M. Lee, W. Christen, S.S. Bassuk, S. Mora, H. Gibson, C.M. Albert, D. Gordon, T. Copeland, D. D'Agostino, G. Friedenberg, C. Ridge, V. Bubes, E.L. Giovannucci, W.C. Willett, J.E. Buring, V.R. Group, Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer, N Engl J Med, (2018).
[107] P.B. Adams, S. Lawson, A. Sanigorski, A.J. Sinclair, Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression, Lipids, 31 Suppl (1996) S157-161.
[108] J.T. Brenna, N. Salem, Jr., A.J. Sinclair, S.C. Cunnane, A. International Society for the Study of Fatty, I. Lipids, alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans, Prostaglandins Leukot Essent Fatty Acids, 80 (2009) 85- 91.
[109] A. Hoge, V. Tabar, A.F. Donneau, N. Dardenne, S. Degee, M. Timmermans, M. Nisolle, M. Guillaume, V. Castronovo, Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort, Nutrients, 11 (2019).
[110] S.E. Carlson, B.J. Gajewski, C.J. Valentine, L.K. Rogers, C.P. Weiner, E.A. DeFranco, C.S. Buhimschi, Assessment of DHA on reducing early preterm birth: the ADORE randomized controlled trial protocol, BMC Pregnancy Childbirth, 17 (2017) 62.
[111] W.H. Organization, Born Too Soon. The Global Action Report on Preterm Birth, https://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf, (2012).
[112] K.A. Mulder, R. Elango, S.M. Innis, Fetal DHA inadequacy and the impact on child neurodevelopment: a follow-up of a randomised trial of maternal DHA supplementation in pregnancy, The British journal of nutrition, 119 (2018) 271-279.
[113] E. Ogundipe, M.R. Johnson, Y. Wang, M.A. Crawford, Peri-conception maternal lipid profiles predict pregnancy outcomes, Prostaglandins Leukot Essent Fatty Acids, 114 (2016) 35- 43.
[114] M.A. Crawford, A.J. Sinclair, Nutritional influences in the evolution of mammalian brain. In: lipids, malnutrition & the developing brain, Ciba Found Symp, (1971) 267-292.
[115] FAOI/WHO, Joint Expert Consultations on the “Role of Dietary Fats in Human nutrition” reports no 3, 57, and 91, FAO, Rome., ( 1997, 1994, 2010).
[116] Y. Xiao, Y. Huang, Z.Y. Chen, Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain, Br J Nutr, 94 (2005) 544-550.
[117] J.T. Brenna, G.Y. Diau, The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition, Prostaglandins Leukot Essent Fatty Acids, 77 (2007) 247-250.
[118] M.A. Crawford, N.M. Casperd, A.J. Sinclair, The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores, Comp Biochem Physiol B, 54 (1976) 395-401.
[119] FAO-WHO, FAO-WHO (1978, 1994, 2008-10). Joint International Consultations on the Role of Dietary Fats in Human Nutrition: Nutrition Reports 3, 69, 91, FAO, Rome. , (1978, 1994, 2008-10).
[120] C.N. Serhan, B.D. Levy, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J Clin Invest, 128 (2018) 2657-2669.
[121] M.A. Crawford, K. Costeloe, K. Ghebremeskel, A. Phylactos, L. Skirvin, F. Stacey, Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies?, Am J Clin Nutr, 66 (1997) 1032S-1041S.
[122] C.E. Ramsden, K.R. Faurot, D. Zamora, O.S. Palsson, B.A. MacIntosh, S. Gaylord, A.Y. Taha, S.I. Rapoport, J.R. Hibbeln, J.M. Davis, J.D. Mann, Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial, Pain, 156 (2015) 587-596.
[123] J.G. Devassy, S. Leng, M. Gabbs, M. Monirujjaman, H.M. Aukema, Omega-3 Polyunsaturated Fatty Acids and Oxylipins in Neuroinflammation and Management of Alzheimer Disease, Adv Nutr, 7 (2016) 905-916.
[124] M. Martinez, Tissue levels of polyunsaturated fatty acids during early human development, J Pediatr, 120 (1992) S129-138.
[125] J.D. Carver, V.J. Benford, B. Han, A.B. Cantor, The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects, Brain Res Bull, 56 (2001) 79-85.
[126] G.Y. Diau, A.T. Hsieh, E.A. Sarkadi-Nagy, V. Wijendran, P.W. Nathanielsz, J.T. Brenna, The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system, BMC Med, 3 (2005) 11.
[127] J. Farquharson, F. Cockburn, W.A. Patrick, E.C. Jamieson, R.W. Logan, Infant cerebral cortex phospholipid fatty-acid composition and diet, Lancet, 340 (1992) 810-813.
[128] R.W. Byard, M. Makrides, M. Need, M.A. Neumann, R.A. Gibson, Sudden infant death syndrome: effect of breast and formula feeding on frontal cortex and brainstem lipid composition, J Paediatr Child Health, 31 (1995) 14-16.
[129] R. Uauy, D.R. Hoffman, P. Mena, A. Llanos, E.E. Birch, Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials, J Pediatr, 143 (2003) S17-25.
[130] G.Y. Diau, E.R. Loew, V. Wijendran, E. Sarkadi-Nagy, P.W. Nathanielsz, J.T. Brenna, Docosahexaenoic and arachidonic acid influence on preterm baboon retinal composition and function, Investigative ophthalmology & visual science, 44 (2003) 4559-4566.
[131] E. Ogundipe, N. Tusor, Y. Wang, M.R. Johnson, A.D. Edwards, M.A. Crawford, Randomized controlled trial of brain specific fatty acid supplementation in pregnant women increases brain volumes on MRI scans of their newborn infants, Prostaglandins Leukot Essent Fatty Acids, 138 (2018) 6-13.
[132] J.P. Rivers, M.A. Crawford, Maternal nutrition and the sex ratio at birth, Nature, 252 (1974) 297-298.
[133] A. Chawla, J.J. Repa, R.M. Evans, D.J. Mangelsdorf, Nuclear receptors and lipid physiology: opening the X-files, Science, 294 (2001) 1866-1870.
[134] C. Bernard, Le ons sur les ph nom nes de la vie communs aux animaux et aux vegetaux, Paris, Bailliere JB, editor. 1878., (1878).
[135] R.W. Smithells, S. Sheppard, J. Wild, C.J. Schorah, Prevention of neural tube defect recurrences in Yorkshire: final report, Lancet, 2 (1989) 498-499.
[136] M.V.S.R. Group., Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group, Lancet, 338 (1991) 131-137.
[137] A.E. Czeizel, I. Dudas, J. Metneki, Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Final report, Arch Gynecol Obstet, 255 (1994) 131-139.
[138] A.E. Czeizel, F. Banhidy, Vitamin supply in pregnancy for prevention of congenital birth defects, Curr Opin Clin Nutr Metab Care, 14 (2011) 291-296.
[139] M.A. Crawford, J.P.W. Rivers, The Protein Myth, In Steele, F., Bourne AG., “The Man Food Equation” Academic Press, London, England., (1975) 235-246.
[140] EveryPreemie, INDIA PROFILE OF PRETERM AND LOW BIRTH WEIGHT, PREVENTION AND CARE., https://www.everypreemie.org/wp-content/uploads/2016/01/India- revJan2016.pdf, (2016).
[141] A.C. Candido, N.S. Morais, L.V. Dutra, C.A. Pinto, S. Franceschini, R.C.G. Alfenas, Insufficient iodine intake in pregnant women in different regions of the world: a systematic review, Arch Endocrinol Metab, 63 (2019) 306-311.
[142] P. Budowski, M.J. Leighfield, M.A. Crawford, Nutritional encephalomalacia in the chick: an exposure of the vulnerable period for cerebellar development and the possible need for both omega 6- and omega 3-fatty acids, Br J Nutr, 58 (1987) 511-520.
[143] D.R. Hoffman, J.A. Boettcher, D.A. Diersen-Schade, Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials, Prostaglandins Leukot Essent Fatty Acids, 81 (2009) 151-158.
[144] K. Krishnaswamy., Dietary Guidelines for India, Chair, Dr Kamala Krishnasamy, NIN and the Council for Medical Research, New Delhi, 2nd Edition, 2011, published by the NIN, Hyderabad, 500 007, India. , (2011).
[145] H. Bhattacharyya, C.K. Nath, S. Pala, G.K. Medhi, H. Chutia, Iodine Deficiency Disorders in Children in East Khasi Hills District of Meghalaya, India, Indian Pediatr, 57 (2020) 811-814.
[146] R.K. Srivastava, http://nhm.gov.in/images/pdf/programmes/ndcp/niddcp/revised_guidelines.pdf, (2006).
[147] R. Rana, R.S. Raghuvanshi, Effect of different cooking methods on iodine losses, J Food Sci Technol, 50 (2013) 1212-1216.
[148] I.S. Hussein, Y. Min, K. Ghebremeskel, A.M. Gaffar, Iodine status and fish intake of Sudanese schoolchildren living in the Red Sea and White Nile regions, Public Health Nutr, 15 (2012) 2265-2271.
[149] K.B. Nyuar, Y. Min, K. Ghebremeskel, A.K. Khalil, M.I. Elbashir, M.A. Cawford, Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid, Acta Paediatr, 99 (2010) 1824-1827.
[150] P.J. Drury, M.A. Crawford, Essential fatty acids in human milk. In: Ballabriga, A., Brunser, 0., Dobbing, J. and Senterre, M.G.J, eds. Clinical Nutrition of the Young Child. Raven Press. New York. , (1990) 289-312.
[151] M. Sas, J.J. Gellen, N. Dusitsin, M. Tunkeyoon, S. Chalapati, M.A. Crawford, P.J. Drury, T. Lenihan, O. Ayeni, A. Pinol, An investigation on the influence of steroidal contraceptives on milk lipid and fatty acids in Hungary and Thailand. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on oral contraceptives, Contraception, 33 (1986) 159-178.
[152] E.N. Smit, E.A. Oelen, E. Seerat, F.A. Muskiet, E.R. Boersma, Breast milk docosahexaenoic acid (DHA) correlates with DHA status of malnourished infants, Arch Dis Child, 82 (2000) 493-494.
[153] J.T. Brenna, B. Varamini, R.G. Jensen, D.A. Diersen-Schade, J.A. Boettcher, L.M. Arterburn, Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide, Am J Clin Nutr, 85 (2007) 1457-1464.
[154] K. Joshi, M. Gadgil, A. Pandit, S. Otiv, K.S.D. Kothapalli, J.T. Brenna, Dietary pattern regulates fatty acid desaturase 1 gene expression in Indian pregnant women to spare overall long chain polyunsaturated fatty acids levels, Mol Biol Rep, 46 (2019) 687-693.
[155] P.c.t.M. Crawford., Patrick Sorgeloos and Vu Ngoc Ut. Personal communication to Michael Crawford. 05/08/2019, (2019).







